CONTROL OF MICROBIAL INFECTIONS
CONTROL OF ZOONOSES:
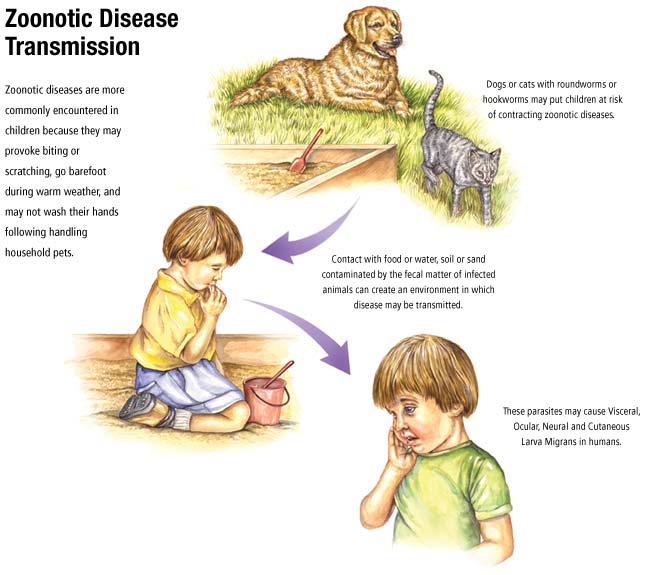
Zoonotic sources of infections will be impossible to eradicate without killing all the animal reservoirs.
Alternative strategies are the only hope and, with over 200 organisms that can cause zoonoses in
humans, control rather than eradication is of great importance. The transmission of many zoonoses from
animals to humans occurs via insect vectors, for example louse-borne infections by Rickettsia
spp. (typhus), tick borne infections by Borrelia spp. (e.g. Lyme’s disease), yellow fever virus is
transmitted by mosquitoes of the genus Aedes, and plague (Yersinia pestis) transmitted by fleas. Perhaps
the most famous zoonosis, rabies is caught directly from the bite of infected animals, typically dogs.
With deforestation and increased expansion of cities (the slum regions, that is!) humans are encountering
arthropod vector-borne diseases more frequently. The displaced vectors are driven from forests into new
geographical locations and the micro-organisms spread into new vectors.
Less shocking than exotic infections such as Ebola virus outbreaks, food acts as the source and vehicle
for transmission of infections such as salmonellosis and campylobacteriosis and thus may be zoonoses. Food products are regulated at several levels but simple hygienic precautions will prevent many infections. Correct storage temperatures will prevent multiplication of microbes and adequate cooking
temperatures will kill organisms. Pasteurisation of milk is an outstanding example of the efficacy of disinfection eliminating a disease; sterilisation of milk at extreme temperature levels is not necessary to kill
Mycobacterium bovis. The control of food-borne disease will depend on removal of the pathogens in the
original foodstuff (e.g. salmonellae in chickens and their feed) as well as maintaining correct handling
and cooking methods. Each of the various steps in the food chain (source, manufacture, distribution and
destination) should offer control points at which microbial contamination can be checked.
CONTROL OF VECTORS
The control of vector-borne disease has significantly greater problems than in directly acquired zoonoses
such as food poisoning. With the increases in the distribution and number of cases of Dengue fever, an
arthropod-borne illness caused by a flavivirus, we have problems similar to those in controlling malaria,
notably controlling the mosquitoes that carry the virus. The mosquitoes that carry dengue virus, Aedes
spp., have spread back into the central Americas having once been eradicated. This means that Dengue
is now the most common arbovirus infection in humans responsible for millions of cases annually. It is
clear that eradication is likely to be impossible for any zoonosis because it will be unlikely that the
reservoir(s) or vectors can be eradicated, especially if the organism is able to reside in a variety of host
and vector species. With dengue virus, the virus is maintained in monkeys and transferred by the
mosquito within forests (hence termed sylvatic cycle). Disturbingly, the virus has widened its vector
range by using the Aedes species that prefer urban environments (Aedes aegypti) such that dengue is
maintained within human populations (the urban cycle). Note then that urban dengue is not a zoonosis).
You should note that with malaria in humans, the species responsible (protozoa of the genus
Plasmodium) are restricted to, and transmitted only between, humans. Malaria is therefore not a
zoonosis. Theoretically at least it is possible to eradicate malaria if you eradicate the vector. With
zoonoses, eradication of the natural host is likely to be impossible. Under such circumstances one can
only hope for controlling the infection rather than eradicating the infection. The term ‘eradication’ is not
used to describe the treatment of an individual patient with antimicrobial drugs, but should be reserved
when discussing the elimination of a disease from a geographical zone (or, famously, smallpox from the
world!).
To stand any chance of controlling vector-borne infections, an understanding of the biology of the vector
itself is essential. As microbiologists, one may slip into thinking that expertise in the virus or bacterium
is sufficient, but the behaviour of the vector is critical in devising strategies of control. For example, the
sixty species of Anopheles mosquito that can transmit malaria to humans differ in their biting times and
preference for breeding sites. The key features include knowledge of their breeding sites, biting behaviour, resting sites and host preferences (animal or human). Female mosquitoes are blood sucking because of the nutritional demands of producing eggs. Males can live off nectar. Wholescale spraying with insecticides such as DDT has been effective in the past, but the problem of the
mosquito developing resistance to the insecticide has been steadily increasing. At first sight DDT
appears to be attractive: stable (it remains active on the walls of sprayed rooms for days) and selectively
toxic; weight for weight the toxicity is greatest against insects than against higher animals.
Unfortunately, DDT has a half life of greater then 100 years and accumulates to toxic levels in the food
chain. The environmental side effects of DDT have led to the more controlled use of DDT and other less
potent chemical insecticides exemplified best in the development of impregnated bed nets. Of the
various biological controls tested the use of a larvacidal toxin has had the most success. Bacillus thuringiensis produces a pore-forming toxin that kills insect larvae and, being a member of the genus Bacillus, produces endospores which makes it feasible to spray large areas with a suspension of the organism in a suitably robust form. Prompt diagnosis and treatment of individual cases of vector-borne diseases will also serve to reduce the opportunities for transmission from infected hosts.
The control of vectors is a national effort with the assistance of the World Health Organisation to coordinate such programmes. The financial resources and political commitment need to be maintained if
any prolonged effects are to be realised. The return of mosquitoes to areas that have been previously
cleared has been witnessed in many areas of the globe. Most vector control programmes aim to reduce
the population size of the vector. Eradication being that much more difficult to sustain, it will usually
stand better chances of success on islands rather than mainland areas.
IMMUNO- AND CHEMOPROPHYLAXIS:
Immunoprophylaxisis the administration of immune serum to people who are considered at acute risk
of developing diseases against which there is a protective anti-serum, for example, tetanus, botulism and
diphtheria. Such protection is effective but of limited scope in that it protects the few people at risk for a
limited period of time but has no effect on the control of the disease in the community. There is little, if
any, distinction between the terms ‘immunoprophylaxis’ and ‘passive vaccination’.
Chemoprophylaxisis the use of antibiotics in two circumstances:
1 . to protect contacts of patients with infectious diseases from developing clinical disease, and
2 . to provide temporary protection during the course of a medical intervention or surgical operation.
Again, chemo-prophylaxis provides cover for the immediate contacts and cannot be employed to provide
prolonged, widespread protection for communities. Antibiotics have not eliminated infectious disease or
infections from the world, nor in fact have they significantly reduced their incidence. It may be argued
that the prophylactic use of antibiotics has prevented infections in specific circumstances such as
outbreaks of meningococcal meningitis in schools. In these circumstances close contacts (e.g. school
children in the same school) are given antibiotics to prevent the disease developing. Immunization will
be inappropriate (assuming there is a suitable vaccine) because the time needed to develop immunity (2–
4 weeks) will leave the contacts unprotected in the short term. Meningococcal meningitis can occur in clusters of people in a pattern consistent with a common source outbreak. As vaccines are not available for all the sero groups of Neisseria meningitidis(antibodies against the polysaccharide capsules are protective) antibiotics are given to prevent the disease developing. Another area where antibiotics have prevented infection is the prophylactic cover for surgical operations. For protection during surgery, the antibiotics need to be broad spectrum and given so that the peak levels are obtained during the operation. Whilst this has not abolished post-operative infections, the incidence of post-operative infections would undoubtedly be higher if such prophylaxis was omitted.
CITED BY KAMAL SINGH KHADKA
MSC MICROBIOLOGY, TU.
ASSISTANT PROFESSOR IN PU, PBPC, PNC.
POKHARA, NEPAL.
SOME SUGGESTED REFERENCES:
www.who.int/zoonoses/control_neglected_zoonoses/en/
www.fao.org/docrep/006/y4962t/y4962t01.htm
www.cdc.gov/24-7/cdcfastfacts/zoonotic.html
www.oie.int/doc/ged/D8633.PDF
www.dshs.state.tx.us › Infectious Disease Control
en.wikipedia.org/wiki/Zoonosis
en.wikipedia.org/wiki/Vector_control
www.who.int/malaria/areas/vector_control/en/
www.cdc.gov/nceh/ehs/topics/vectorcontrol.htm
Zoonotic sources of infections will be impossible to eradicate without killing all the animal reservoirs.
Alternative strategies are the only hope and, with over 200 organisms that can cause zoonoses in
humans, control rather than eradication is of great importance. The transmission of many zoonoses from
animals to humans occurs via insect vectors, for example louse-borne infections by Rickettsia
spp. (typhus), tick borne infections by Borrelia spp. (e.g. Lyme’s disease), yellow fever virus is
transmitted by mosquitoes of the genus Aedes, and plague (Yersinia pestis) transmitted by fleas. Perhaps
the most famous zoonosis, rabies is caught directly from the bite of infected animals, typically dogs.
With deforestation and increased expansion of cities (the slum regions, that is!) humans are encountering
arthropod vector-borne diseases more frequently. The displaced vectors are driven from forests into new
geographical locations and the micro-organisms spread into new vectors.
Less shocking than exotic infections such as Ebola virus outbreaks, food acts as the source and vehicle
for transmission of infections such as salmonellosis and campylobacteriosis and thus may be zoonoses. Food products are regulated at several levels but simple hygienic precautions will prevent many infections. Correct storage temperatures will prevent multiplication of microbes and adequate cooking
temperatures will kill organisms. Pasteurisation of milk is an outstanding example of the efficacy of disinfection eliminating a disease; sterilisation of milk at extreme temperature levels is not necessary to kill
Mycobacterium bovis. The control of food-borne disease will depend on removal of the pathogens in the
original foodstuff (e.g. salmonellae in chickens and their feed) as well as maintaining correct handling
and cooking methods. Each of the various steps in the food chain (source, manufacture, distribution and
destination) should offer control points at which microbial contamination can be checked.
CONTROL OF VECTORS
The control of vector-borne disease has significantly greater problems than in directly acquired zoonoses
such as food poisoning. With the increases in the distribution and number of cases of Dengue fever, an
arthropod-borne illness caused by a flavivirus, we have problems similar to those in controlling malaria,
notably controlling the mosquitoes that carry the virus. The mosquitoes that carry dengue virus, Aedes
spp., have spread back into the central Americas having once been eradicated. This means that Dengue
is now the most common arbovirus infection in humans responsible for millions of cases annually. It is
clear that eradication is likely to be impossible for any zoonosis because it will be unlikely that the
reservoir(s) or vectors can be eradicated, especially if the organism is able to reside in a variety of host
and vector species. With dengue virus, the virus is maintained in monkeys and transferred by the
mosquito within forests (hence termed sylvatic cycle). Disturbingly, the virus has widened its vector
range by using the Aedes species that prefer urban environments (Aedes aegypti) such that dengue is
maintained within human populations (the urban cycle). Note then that urban dengue is not a zoonosis).
You should note that with malaria in humans, the species responsible (protozoa of the genus
Plasmodium) are restricted to, and transmitted only between, humans. Malaria is therefore not a
zoonosis. Theoretically at least it is possible to eradicate malaria if you eradicate the vector. With
zoonoses, eradication of the natural host is likely to be impossible. Under such circumstances one can
only hope for controlling the infection rather than eradicating the infection. The term ‘eradication’ is not
used to describe the treatment of an individual patient with antimicrobial drugs, but should be reserved
when discussing the elimination of a disease from a geographical zone (or, famously, smallpox from the
world!).
To stand any chance of controlling vector-borne infections, an understanding of the biology of the vector
itself is essential. As microbiologists, one may slip into thinking that expertise in the virus or bacterium
is sufficient, but the behaviour of the vector is critical in devising strategies of control. For example, the
sixty species of Anopheles mosquito that can transmit malaria to humans differ in their biting times and
preference for breeding sites. The key features include knowledge of their breeding sites, biting behaviour, resting sites and host preferences (animal or human). Female mosquitoes are blood sucking because of the nutritional demands of producing eggs. Males can live off nectar. Wholescale spraying with insecticides such as DDT has been effective in the past, but the problem of the
mosquito developing resistance to the insecticide has been steadily increasing. At first sight DDT
appears to be attractive: stable (it remains active on the walls of sprayed rooms for days) and selectively
toxic; weight for weight the toxicity is greatest against insects than against higher animals.
Unfortunately, DDT has a half life of greater then 100 years and accumulates to toxic levels in the food
chain. The environmental side effects of DDT have led to the more controlled use of DDT and other less
potent chemical insecticides exemplified best in the development of impregnated bed nets. Of the
various biological controls tested the use of a larvacidal toxin has had the most success. Bacillus thuringiensis produces a pore-forming toxin that kills insect larvae and, being a member of the genus Bacillus, produces endospores which makes it feasible to spray large areas with a suspension of the organism in a suitably robust form. Prompt diagnosis and treatment of individual cases of vector-borne diseases will also serve to reduce the opportunities for transmission from infected hosts.
The control of vectors is a national effort with the assistance of the World Health Organisation to coordinate such programmes. The financial resources and political commitment need to be maintained if
any prolonged effects are to be realised. The return of mosquitoes to areas that have been previously
cleared has been witnessed in many areas of the globe. Most vector control programmes aim to reduce
the population size of the vector. Eradication being that much more difficult to sustain, it will usually
stand better chances of success on islands rather than mainland areas.
IMMUNO- AND CHEMOPROPHYLAXIS:
Immunoprophylaxisis the administration of immune serum to people who are considered at acute risk
of developing diseases against which there is a protective anti-serum, for example, tetanus, botulism and
diphtheria. Such protection is effective but of limited scope in that it protects the few people at risk for a
limited period of time but has no effect on the control of the disease in the community. There is little, if
any, distinction between the terms ‘immunoprophylaxis’ and ‘passive vaccination’.
Chemoprophylaxisis the use of antibiotics in two circumstances:
1 . to protect contacts of patients with infectious diseases from developing clinical disease, and
2 . to provide temporary protection during the course of a medical intervention or surgical operation.
Again, chemo-prophylaxis provides cover for the immediate contacts and cannot be employed to provide
prolonged, widespread protection for communities. Antibiotics have not eliminated infectious disease or
infections from the world, nor in fact have they significantly reduced their incidence. It may be argued
that the prophylactic use of antibiotics has prevented infections in specific circumstances such as
outbreaks of meningococcal meningitis in schools. In these circumstances close contacts (e.g. school
children in the same school) are given antibiotics to prevent the disease developing. Immunization will
be inappropriate (assuming there is a suitable vaccine) because the time needed to develop immunity (2–
4 weeks) will leave the contacts unprotected in the short term. Meningococcal meningitis can occur in clusters of people in a pattern consistent with a common source outbreak. As vaccines are not available for all the sero groups of Neisseria meningitidis(antibodies against the polysaccharide capsules are protective) antibiotics are given to prevent the disease developing. Another area where antibiotics have prevented infection is the prophylactic cover for surgical operations. For protection during surgery, the antibiotics need to be broad spectrum and given so that the peak levels are obtained during the operation. Whilst this has not abolished post-operative infections, the incidence of post-operative infections would undoubtedly be higher if such prophylaxis was omitted.
CITED BY KAMAL SINGH KHADKA
MSC MICROBIOLOGY, TU.
ASSISTANT PROFESSOR IN PU, PBPC, PNC.
POKHARA, NEPAL.
SOME SUGGESTED REFERENCES:
www.who.int/zoonoses/control_neglected_zoonoses/en/
www.fao.org/docrep/006/y4962t/y4962t01.htm
www.cdc.gov/24-7/cdcfastfacts/zoonotic.html
www.oie.int/doc/ged/D8633.PDF
www.dshs.state.tx.us › Infectious Disease Control
en.wikipedia.org/wiki/Zoonosis
en.wikipedia.org/wiki/Vector_control
www.who.int/malaria/areas/vector_control/en/
www.cdc.gov/nceh/ehs/topics/vectorcontrol.htm






Comments