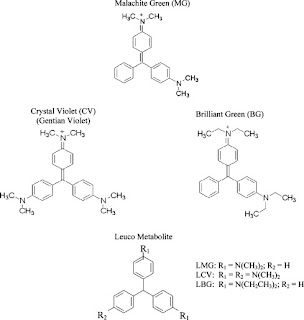
CONTROL OF MOS BY CHEMICAL AGENTS CONTD..

G) QUATS (QUATERNARY AMMONIUM COMPOUNDS) : They are germicidal, cationic detergents. They are bactericidal against Gran positive bacteria & Gram negative bacteria & are also effective against fungi to certain pathogenic protozoa. Virus appear to be more resistant than bacteria. The two popular quats are Zephiran, a brand mane of benzalkonium chloride & Cepacol, a brand name of cetylpyridinium chloride.Eg Cetrimide . Please visit this link;http://en.wikipedia.org/wiki/Disinfectant Uses: They are used as skin disinfectant, preservatives in ophthalmic solution,& cosmetic preparations. They are widely used for control of mos on floors & walls of hospitals & public places. They are also used to sanitize food & beverage utensils in the restaurant & in food processing plants. Mode of Action: They denature protein, interfere with glycolysis & membrane damage. They mostly damage the cytoplasmic membrane by altering the permeability. T