DIAGNOSTIC MEDICAL MICROBIOLOGY
PRINCIPLES OF DIAGNOSTIC MICROBIOLOGY...
CULTURE SYSTEMS:
For diagnostic bacteriology , it is necessary to use several types of media for routine culture, particularly when the possible organisms include aerobic, facultatively anaerobic, and obligately anaerobic bacteria. The specimens and culture media used to diagnose the more common bacterial infections.
The standard medium for specimens is blood agar , usually made with 5% sheep blood. Most aerobic and
facultatively anaerobic organisms will grow on blood agar . Chocolate agar , a medium containing heated blood with or without supplements, is a second necessary medium; some organisms that do not grow on blood agar,including pathogenic Neisseria and Haemophilus, will grow on chocolate agar. A selective medium for enteric gram-negative rods (either MacConkey agar or eosin-methylene blue [EMB] agar) is a third type of medium used routinely. Specimens to be cultured for obligate anaerobes must be plated on at least two additional types of media, including a highly supplemented agar such as brucella agar with hemin and vitamin K and a selective medium containing substances that inhibit the growth of enteric gram-negative rods and facultatively anaerobic or anaerobic gram-positive cocci.
CULTURE SYSTEMS:
For diagnostic bacteriology , it is necessary to use several types of media for routine culture, particularly when the possible organisms include aerobic, facultatively anaerobic, and obligately anaerobic bacteria. The specimens and culture media used to diagnose the more common bacterial infections.
The standard medium for specimens is blood agar , usually made with 5% sheep blood. Most aerobic and
facultatively anaerobic organisms will grow on blood agar . Chocolate agar , a medium containing heated blood with or without supplements, is a second necessary medium; some organisms that do not grow on blood agar,including pathogenic Neisseria and Haemophilus, will grow on chocolate agar. A selective medium for enteric gram-negative rods (either MacConkey agar or eosin-methylene blue [EMB] agar) is a third type of medium used routinely. Specimens to be cultured for obligate anaerobes must be plated on at least two additional types of media, including a highly supplemented agar such as brucella agar with hemin and vitamin K and a selective medium containing substances that inhibit the growth of enteric gram-negative rods and facultatively anaerobic or anaerobic gram-positive cocci.
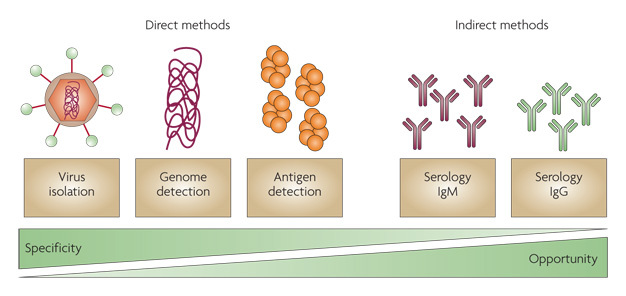
Antigen Detection:
Immunologic systems designed to detect antigens of microorganisms can be used in the diagnosis of specific
infections. IF tests (direct and indirect fluorescent antibody tests) are one form of antigen detection and are
discussed in separate sections in this blog on the diagnosis of bacterial, chlamydial, and viral infections.
EIAs, including enzyme-linked immunosorbent assays (ELISA), and agglutination tests are used to detect
antigens of infectious agents present in clinical specimens. The principles of these tests are reviewed briefly
here.
There are many variations of EIAs to detect antigens. One commonly used format is to bind a capture antibody, specific for the antigen in question, to the wells of plastic microdilution trays. The specimen containing the antigen is incubated in the wells followed by washing of the wells. A second antibody for the antigen, labeled with enzyme, is used to detect the antigen. Addition of the substrate for the enzyme allows detection of the bound antigen by colorimetric reaction. A significant modification of EIAs is the development of immunochromatographic membrane formats for antigen detection. In this format, a nitrocellulose membrane is used to absorb the antigen from a specimen. A colored reaction appears directly on the membrane with sequential addition of conjugate followed by substrate. In some formats, the antigen is captured by bound antibody directed against the antigen. These assays have the advantage of being rapid and also frequently include a built-in positive control. An example of this type of assay is the Binax NOW Streptococcus pneumoniae urinary antigen test. In some EIAs, the initial antibody is not necessary , because the antigen will bind directly to the plastic of the wells. EIAs are used to detect viral, bacterial, chlamydial, protozoan, and fungal antigens in a variety of specimen types such as stool, cerebrospinal fluid, urine, and respiratory samples.
In latex agglutination tests, an antigen-specific antibody (either polyclonal or monoclonal) is fixed to latex
beads. When the clinical specimen is added to a suspension of the latex beads, the antibodies bind to the
antigens on the microorganism forming a lattice structure, and agglutination of the beads occurs.
Coagglutination is similar to latex agglutination except that staphylococci rich in protein A (Cowan I strain) are used instead of latex particles; coagglutination is less useful for antigen detection compared with latex
agglutination but is helpful when applied to identification of bacteria in cultures such as S pneumoniae, Neisseria meningitidis, N gonorrhoeae, and Beta-hemolytic streptococci. Latex agglutination tests are primarily directed at the detection of carbohydrate antigens of encapsulated microorganisms. Antigen detection is used most often in the diagnosis of group A streptococcal pharyngitis. Detection of cryptococcal antigen is useful in the diagnosis of cryptococcal meningitis in patients with AIDS or other immunosuppressive diseases. The sensitivity of latex agglutination tests in the diagnosis of bacterial meningitis may not be better than that of Gram stain, which is approximately 100,000 bacteria per milliliter . For that reason, the latex agglutination test is not recommended for direct specimen testing.
Western Blot Immunoassays
These assays are usually performed to detect antibodies against specific antigens of a particular organism. This method is based upon the electrophoretic separation of major proteins of the organism in question in a
twodimensional agarose gel. Organisms are mechanically or chemically disrupted and resultant solubilized antigen of the organism is placed in a polyacrylamide gel. An electric current is applied and major proteins are separated out on the basis of size (smaller proteins travel faster). The protein bands are transferred to strips of nitrocellulose paper . Following incubation of the strips with a patient's specimen containing antibody (usually serum), the antibodies bind to the proteins on the strip and are detected enzymatically in a fashion similar to the EIA methods described above. Western blot tests are used as specific tests for antibodies in HIV infection and Lyme disease.
Molecular Diagnostics
The principle behind early molecular assays is the hybridization of a characterized nucleic acid probe to a
specific nucleic acid sequence in a test specimen followed by detection of the paired hybrid. F or example,
single-stranded probe DNA (or RNA) is used to detect complementary RNA or denatured DNA in a test
specimen. The nucleic acid probe typically is labeled with enzymes, antigenic substrates, chemiluminescent
molecules, or radioisotopes to facilitate detection of the hybridization product. By carefully selecting the probe or making a specific oligonucleotide and performing the hybridization under conditions of high stringency,detection of the nucleic acid in the test specimen can be extremely specific. Such assays are currently used primarily for rapid confirmation of a pathogen once growth is detected, eg, the identification of Mycobacterium tuberculosis in culture using the Gen-Probe Inc. (San Diego, CA) DNA probe. The Gen-Probe test is an example of a hybridization test format in which the probe and target are in solution. Most of the applications in use in clinical microbiology laboratories make use of solution hybridization formats. In situ hybridization involves the use of labeled DNA probes or labeled RNA probes to detect complementary nucleic acids in formalin-fixed paraffin-embedded tissues, frozen tissues, or cytologic preparations mounted on slides. Technically, this can be difficult and is usually performed in histology laboratories and not clinical microbiology laboratories. However,this technique has increased the knowledge of the biology of many infectious diseases, especially the hepatitides and oncogenic viruses, and is still useful in infectious diseases diagnosis.A novel technique that is somewhat of a modification of in situ hybridization makes use of peptide nucleic acid probes. Peptide nucleic acid probes are synthesized pieces of DNA in which the sugar phosphate backbone of DNA (normally negatively charged) is replaced by a polyamide of repetitive units (neutral charge). Individual nucleotide bases can be attached to the now neutral backbone, which allows for faster and more specific hybridization to complementary nucleic acids. Because the probes are synthetic, they are not subject to degradation by nucleases and other enzymes. A commercial company (AdvanDx, Woburn MA) has a number of FDA cleared assays for confirmation of Staphylococcus aureus, enterococci, certain Candida sp. and some gram-negative bacilli in positive blood culture bottles. The probe hybridization is detected by fluorescence and is called Peptide Nucleic Acid-Fluorescence In Situ Hybridization (PNA -FISH).
Cited By Anil Bhujel
Bsc Microbiology, TU.
Microbiology Student At Pokhara Bigyan Tatha Prabidhi Campus(PBPC), Nayabazzar-9, Pokhara.
SOME SUGGESTED REFERENCES:












Comments