CONTROL OF MICROBIAL INFECTIONS
ANTIBIOTIC RESISTANCE(BACTERIA):
The most pressing problem facing medicine is the growing number of micro-organisms that are now
resistant to a wide range of antibiotics. The term antibiotic resistance describes the condition where
bacteria have developed or acquired means to overcome the inhibitory effect of one or more antibiotics. A chilling example is multidrug-resistant Mycobacterium tuberculosis. Without the development of new antibiotics, thoracotomy (removal of the affected lobes of the lung) appears to be
the only treatment option. The mechanisms by which bacteria develops resistance to antibiotics are either due to mutation or acquisition of resistance genes from other organisms.
The most pressing problem facing medicine is the growing number of micro-organisms that are now
resistant to a wide range of antibiotics. The term antibiotic resistance describes the condition where
bacteria have developed or acquired means to overcome the inhibitory effect of one or more antibiotics. A chilling example is multidrug-resistant Mycobacterium tuberculosis. Without the development of new antibiotics, thoracotomy (removal of the affected lobes of the lung) appears to be
the only treatment option. The mechanisms by which bacteria develops resistance to antibiotics are either due to mutation or acquisition of resistance genes from other organisms.
MUTATION
Clinically, mutation leading to resistance has not been a major problem compared with the acquisition of
resistance genes. In general, the effect of mutation will be to modify the target protein such that the
binding affinity of the antibiotic is reduced. The protein will tolerate a certain loss of efficiency due to
mutations, but constraints will limit the number and frequency of viable mutations in the active site. The
affinity of binding will fall, but not such that the protein function is lost completely. If the binding site
for the antibiotic is distinct from the active site of the protein, then there will be more scope for mutation
to occur without significant loss of function. For example, the binding of an antibiotic on a ribosome
might sterically hinder the correct binding of the tRNA and lead to misreading. If the antibiotic
competed with the tRNA for the same binding site, then mutation of the tRNA binding site will limit the
extent of mutation. Mutational events reduce the potency of the antibiotic for the target site and this is observed by small increases in the concentration of antibiotic needed to inhibit the growth of the organism (minimum inhibitory concentration, MIC); for example, the MIC of Staphylococcus aureus to penicillin may increase from 0.01 µg/ml to 0.05 µg/mL. By comparison, acquisition of
resistance genes usually results in large increases in the MIC (e.g. from 1 µg/ml to >64 µg/mL).
An example of mutational resistance of clinical importance is the resistance of Mycobacterium
tuberculosis to the aminoglycoside streptomycin. During the treatment of patients with tuberculosis, the
proportion of bacteria that are resistant to the effects of streptomycin increases steadily with time due to
mutations in the ribosomal binding site for streptomycin. The frequency of mutation of Mycobacterium
tuberculosis is approximately 1 in 10^7 , meaning that one in every 10 million cells undergoes a
mutational event that affects streptomycin activity. The mutation rate is fixed and therefore occurs
irrespective of the presence of streptomycin. Thus, in patients taking streptomycin, the selective pressure
results in the resistant mutants continuing to grow (unlike the susceptible cells), displacing the falling
numbers of streptomycin susceptible cells until eventually all the bacteria will be resistant to
streptomycin. If the antibiotic is withdrawn then the selective pressure vanishes and the susceptible cells
will regain dominance because of the cost of streptomycin resistance in terms of reduced efficiency of
ribosomal function. The key feature here is the constant rate of mutation; all that changes is the selective
pressure on the organism by exposing the organisms to streptomycin.
Mutational resistance that causes problems for the treatment of a bacterial infection is largely restricted
to Mycobacterium tuberculosis.Why?
The organism has a long generation time (2–4 hours) compared with ‘fast’ growing organisms such as
Esch. coli with a generation time of 20–30 minutes and Escherichia coli has multiple copies of the
rRNA gene whereas Mycobacterium tuberculosis has only one. Any mutation in one of the appropriate
genes in Escherichia coli will be recessive to the unaffected gene copies whereas mutations in the single
copy of the gene in Mycobacterium tuberculosis will be expressed.
ACQUISITION OF RESISTANCE GENES (HORIZONTAL GENE TRANSFER):
Resistance genes are thought to exist in populations of bacteria that will be exposed to antibiotics in their
natural environment. Bacteria in the soil will encounter antibiotics produced by fungi and Streptomyces
spp. The strong selection pressures exerted by antibiotic use in agriculture (animal feeds, crop spraying,
etc.), hospitals and the home (in countries where antibiotics can be bought freely at the pharmacy
without prescription) has resulted in bacterial pathogens acquiring resistance genes from other
organisms. The horizontal gene exchange of transmissible plasmids containing resistance genes means
that effective antibiotic resistance is acquired in one step rather than waiting for mutational events to
develop. The resistance genes have become widespread throughout different pathogens across the globe.
Their mobility is conferred by becoming incorporated in transposons that, in turn, accumulate as gene
cassettes in mobile plasmids called multidrug resistance plasmids(often abbreviated to R plasmids). They resemble the pattern observed with pathogenicity islands, clusters of genes all coding for related functions.
Mutational events may be the only option for organisms that do not readily exchange genes horizontally.
Again, Mycobacterium tuberculosis is a good example. The cell wall structure of Mycobacterium
tuberculosis has the extra mycolic acid/arabinogalactan layers that may prevent transformation,
conjugation and transduction.
Clinically, mutation leading to resistance has not been a major problem compared with the acquisition of
resistance genes. In general, the effect of mutation will be to modify the target protein such that the
binding affinity of the antibiotic is reduced. The protein will tolerate a certain loss of efficiency due to
mutations, but constraints will limit the number and frequency of viable mutations in the active site. The
affinity of binding will fall, but not such that the protein function is lost completely. If the binding site
for the antibiotic is distinct from the active site of the protein, then there will be more scope for mutation
to occur without significant loss of function. For example, the binding of an antibiotic on a ribosome
might sterically hinder the correct binding of the tRNA and lead to misreading. If the antibiotic
competed with the tRNA for the same binding site, then mutation of the tRNA binding site will limit the
extent of mutation. Mutational events reduce the potency of the antibiotic for the target site and this is observed by small increases in the concentration of antibiotic needed to inhibit the growth of the organism (minimum inhibitory concentration, MIC); for example, the MIC of Staphylococcus aureus to penicillin may increase from 0.01 µg/ml to 0.05 µg/mL. By comparison, acquisition of
resistance genes usually results in large increases in the MIC (e.g. from 1 µg/ml to >64 µg/mL).
An example of mutational resistance of clinical importance is the resistance of Mycobacterium
tuberculosis to the aminoglycoside streptomycin. During the treatment of patients with tuberculosis, the
proportion of bacteria that are resistant to the effects of streptomycin increases steadily with time due to
mutations in the ribosomal binding site for streptomycin. The frequency of mutation of Mycobacterium
tuberculosis is approximately 1 in 10^7 , meaning that one in every 10 million cells undergoes a
mutational event that affects streptomycin activity. The mutation rate is fixed and therefore occurs
irrespective of the presence of streptomycin. Thus, in patients taking streptomycin, the selective pressure
results in the resistant mutants continuing to grow (unlike the susceptible cells), displacing the falling
numbers of streptomycin susceptible cells until eventually all the bacteria will be resistant to
streptomycin. If the antibiotic is withdrawn then the selective pressure vanishes and the susceptible cells
will regain dominance because of the cost of streptomycin resistance in terms of reduced efficiency of
ribosomal function. The key feature here is the constant rate of mutation; all that changes is the selective
pressure on the organism by exposing the organisms to streptomycin.
Mutational resistance that causes problems for the treatment of a bacterial infection is largely restricted
to Mycobacterium tuberculosis.Why?
The organism has a long generation time (2–4 hours) compared with ‘fast’ growing organisms such as
Esch. coli with a generation time of 20–30 minutes and Escherichia coli has multiple copies of the
rRNA gene whereas Mycobacterium tuberculosis has only one. Any mutation in one of the appropriate
genes in Escherichia coli will be recessive to the unaffected gene copies whereas mutations in the single
copy of the gene in Mycobacterium tuberculosis will be expressed.
ACQUISITION OF RESISTANCE GENES (HORIZONTAL GENE TRANSFER):
Resistance genes are thought to exist in populations of bacteria that will be exposed to antibiotics in their
natural environment. Bacteria in the soil will encounter antibiotics produced by fungi and Streptomyces
spp. The strong selection pressures exerted by antibiotic use in agriculture (animal feeds, crop spraying,
etc.), hospitals and the home (in countries where antibiotics can be bought freely at the pharmacy
without prescription) has resulted in bacterial pathogens acquiring resistance genes from other
organisms. The horizontal gene exchange of transmissible plasmids containing resistance genes means
that effective antibiotic resistance is acquired in one step rather than waiting for mutational events to
develop. The resistance genes have become widespread throughout different pathogens across the globe.
Their mobility is conferred by becoming incorporated in transposons that, in turn, accumulate as gene
cassettes in mobile plasmids called multidrug resistance plasmids(often abbreviated to R plasmids). They resemble the pattern observed with pathogenicity islands, clusters of genes all coding for related functions.
Mutational events may be the only option for organisms that do not readily exchange genes horizontally.
Again, Mycobacterium tuberculosis is a good example. The cell wall structure of Mycobacterium
tuberculosis has the extra mycolic acid/arabinogalactan layers that may prevent transformation,
conjugation and transduction.
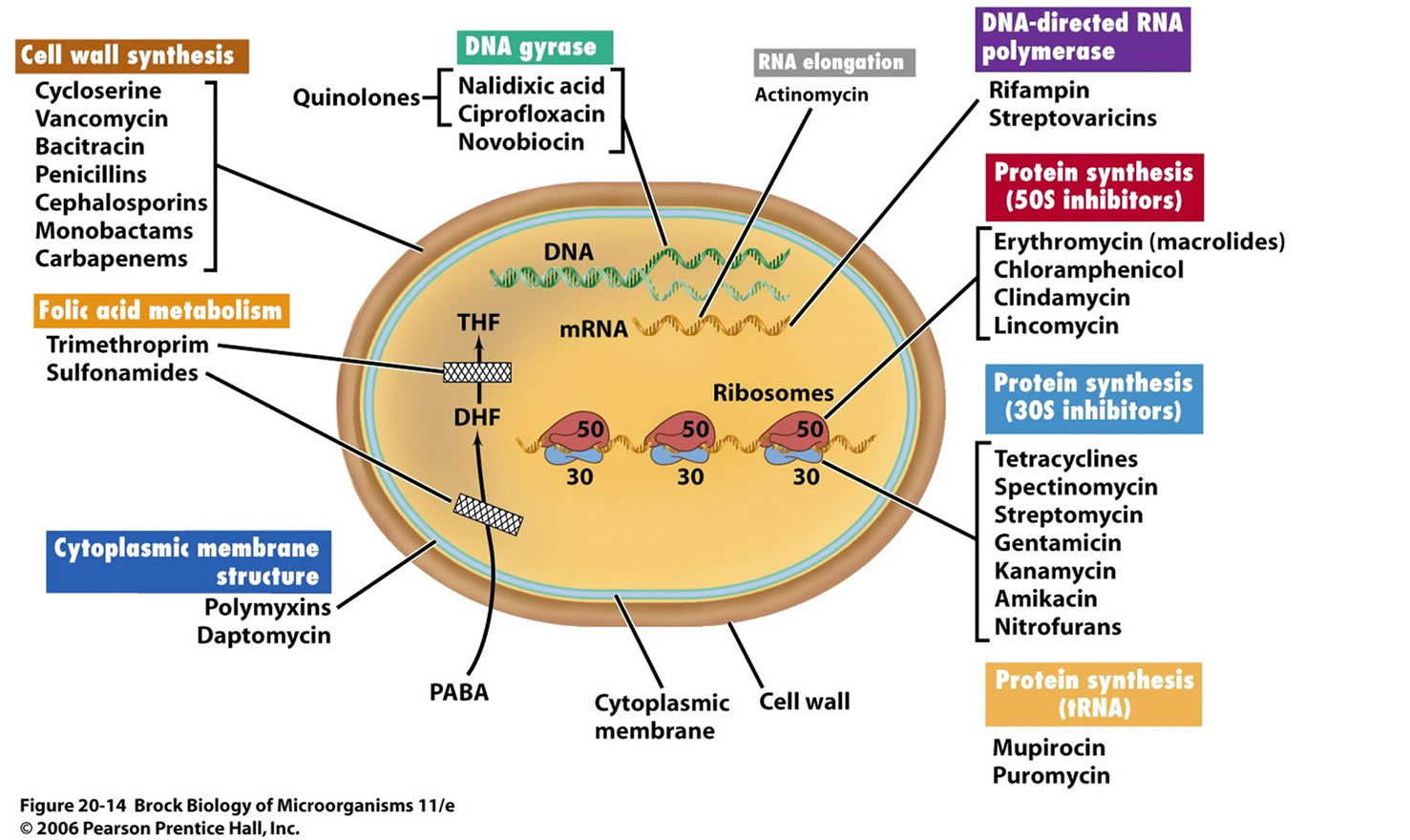
RESISTANCE TO ANTIBIOTICS: CELLULAR MECHANISMS:
Enzymatic inactivation of the antibiotic:
A good example is the production of β-lactamase, an enzyme that hydrolyses the β-lactam nucleus of β-lactam antibiotics (penicillins). These enzymes may be found on the chromosome or on resistance
plasmids and are expressed constitutively or as an inducible enzyme, only when β-lactams are present.
The strategy adopted is exemplified in the Gram positive organism Staphylococcus aureus which
produces inducible β-lactamase to be released extracellularly. In contrast the Gram negative Escherichia
coli constitutively produces much smaller amounts of β-lactamase but sites it in the periplasmic space.
In this way Escherichia coli chooses to only degrade β-lactam antibiotic that permeates through the
porins in the outer membrane. Inactivating enzymes are also found that attack aminoglycosides and
chloramphenicol.
Active efflux of antibiotic:
The bacterium expends energy to actively extrude antibiotic from within the bacterial cell. The efflux
pumps responsible may be able to pump more than one type of antibiotic (multidrug resistance efflux
pumps).
Reduced uptake:
An alternative to removing antibiotic once it has reached the cytosol is to mutate the mechanisms that
are responsible for the uptake of the antibiotic in the first place. Such a strategy relies on active, i.e.
energy expending, mechanisms of uptake. Passive diffusion will not apply.
Modification of drug target:
Mutations will result in alterations of the binding sites in antibiotic target proteins. Penicillin binding
proteins, for example, can reduce their binding affinity for -lactam antibiotics. Alterations in the amino
acid sequences of the ribosomal proteins can also result in reduced binding of the antibiotics that act on
protein synthesis.
Overproduction of target:
With antibiotics such as the sulphonamides and trimethoprim, competitive inhibitors of enzymes
involved in the biosynthesis of folic acid, it is logical to see how bacteria have developed resistance to
these drugs by simply overproducing enzymes. In the case of trimethoprim, the organism produces excess dihydrofolate reductase in order to overcome the competition from the drug.
Some of these mechanisms are produced by mutation, others are simply acquired through horizontal
gene transfer. The origins of the resistance mechanisms lie in other organisms, presumably those that
produce antibiotics themselves. When an organism is under prolonged pressure through exposure to
antibiotics, in time, the appropriate resistance mechanism will eventually make itself available.
It is important to realise that bacteria can be ‘resistant’ to the inhibitory effects of antibiotics through
mechanisms that are neither mutational events nor resulting from acquisition of exogenous resistance
genes.
• Antibiotics have a particular spectrum of activity. Those organisms that are unaffected by a
particular antibiotic may lack the target site or the mechanisms by which the antibiotic enters the
bacterium.
• Even though several antibiotics are bactericidal (e.g. β-lactams), they can only exert this effect on
actively growing bacteria; the organisms in stationary phase are not killed. This is a general
phenomenon and applies not just to antibiotics but to disinfectants and other antibacterial
conditions (starvation, drought). Endospores represent the extreme example of this strategy:
completely inert structures that dominate in unfavourable conditions that are effectively unaffected
by antibiotics.
• Dense collections of bacteria as biofilms offer protection partially through physical restriction and
binding of the antibiotic so as to reduce the active concentration that can penetrate the biofilm.
Cited By Anil Bhujel
Bsc Microbiology, TU.
Microbiology Student At Pokhara Bigyan Tatha Prabidhi Campus, Nayabazzar-9, Pokhara.
SOME SUGGESTED REFERENCES:
en.wikipedia.org/wiki/Antibiotic_resistance
www.sciencedaily.com/articles/a/antibiotic_resistance.htm
textbookofbacteriology.net/resantimicrobial.html
www.who.int/mediacentre/factsheets/fs194/en/
www.rxlist.com/antibiotic_resistance-page3/drugs-condition.htm
textbookofbacteriology.net/resantimicrobial_3.html
www.ncbi.nlm.nih.gov/pubmed/21822035
www.life.umd.edu/classroom/.../MechanismsofAntibioticResistance.htm
www.sciencedirect.com/science/article/pii/S009286740700311X
Enzymatic inactivation of the antibiotic:
A good example is the production of β-lactamase, an enzyme that hydrolyses the β-lactam nucleus of β-lactam antibiotics (penicillins). These enzymes may be found on the chromosome or on resistance
plasmids and are expressed constitutively or as an inducible enzyme, only when β-lactams are present.
The strategy adopted is exemplified in the Gram positive organism Staphylococcus aureus which
produces inducible β-lactamase to be released extracellularly. In contrast the Gram negative Escherichia
coli constitutively produces much smaller amounts of β-lactamase but sites it in the periplasmic space.
In this way Escherichia coli chooses to only degrade β-lactam antibiotic that permeates through the
porins in the outer membrane. Inactivating enzymes are also found that attack aminoglycosides and
chloramphenicol.
Active efflux of antibiotic:
The bacterium expends energy to actively extrude antibiotic from within the bacterial cell. The efflux
pumps responsible may be able to pump more than one type of antibiotic (multidrug resistance efflux
pumps).
Reduced uptake:
An alternative to removing antibiotic once it has reached the cytosol is to mutate the mechanisms that
are responsible for the uptake of the antibiotic in the first place. Such a strategy relies on active, i.e.
energy expending, mechanisms of uptake. Passive diffusion will not apply.
Modification of drug target:
Mutations will result in alterations of the binding sites in antibiotic target proteins. Penicillin binding
proteins, for example, can reduce their binding affinity for -lactam antibiotics. Alterations in the amino
acid sequences of the ribosomal proteins can also result in reduced binding of the antibiotics that act on
protein synthesis.
Overproduction of target:
With antibiotics such as the sulphonamides and trimethoprim, competitive inhibitors of enzymes
involved in the biosynthesis of folic acid, it is logical to see how bacteria have developed resistance to
these drugs by simply overproducing enzymes. In the case of trimethoprim, the organism produces excess dihydrofolate reductase in order to overcome the competition from the drug.
Some of these mechanisms are produced by mutation, others are simply acquired through horizontal
gene transfer. The origins of the resistance mechanisms lie in other organisms, presumably those that
produce antibiotics themselves. When an organism is under prolonged pressure through exposure to
antibiotics, in time, the appropriate resistance mechanism will eventually make itself available.
It is important to realise that bacteria can be ‘resistant’ to the inhibitory effects of antibiotics through
mechanisms that are neither mutational events nor resulting from acquisition of exogenous resistance
genes.
• Antibiotics have a particular spectrum of activity. Those organisms that are unaffected by a
particular antibiotic may lack the target site or the mechanisms by which the antibiotic enters the
bacterium.
• Even though several antibiotics are bactericidal (e.g. β-lactams), they can only exert this effect on
actively growing bacteria; the organisms in stationary phase are not killed. This is a general
phenomenon and applies not just to antibiotics but to disinfectants and other antibacterial
conditions (starvation, drought). Endospores represent the extreme example of this strategy:
completely inert structures that dominate in unfavourable conditions that are effectively unaffected
by antibiotics.
• Dense collections of bacteria as biofilms offer protection partially through physical restriction and
binding of the antibiotic so as to reduce the active concentration that can penetrate the biofilm.
Cited By Anil Bhujel
Bsc Microbiology, TU.
Microbiology Student At Pokhara Bigyan Tatha Prabidhi Campus, Nayabazzar-9, Pokhara.
SOME SUGGESTED REFERENCES:
en.wikipedia.org/wiki/Antibiotic_resistance
www.sciencedaily.com/articles/a/antibiotic_resistance.htm
textbookofbacteriology.net/resantimicrobial.html
www.who.int/mediacentre/factsheets/fs194/en/
www.rxlist.com/antibiotic_resistance-page3/drugs-condition.htm
textbookofbacteriology.net/resantimicrobial_3.html
www.ncbi.nlm.nih.gov/pubmed/21822035
www.life.umd.edu/classroom/.../MechanismsofAntibioticResistance.htm
www.sciencedirect.com/science/article/pii/S009286740700311X









Comments