MICROBIAL BIOTECHNOLOGY: SCOPE, TECHNIQUES CONTD
FOOD TECHNOLOGY:
MONENSIN
Monensin is the most widely used compound fed to cattle to increase feed
efficiency. In feedlot cattle, a dosage of 350 mg/day led to an improvement
in feed efficiency of approximately 6%. In grazing cattle, the average daily
gain increased by 15%. Monensin produces these outcomes by changing the
makeup of the bacterial population in the rumen, thereby influencing the
balance of the end products of ruminal fermentation metabolism.
Monensin is produced by the bacterium Streptomyces cinnamonensis. It is a member of a large and important class of polyketides, the poly ether ionophores. The compound is toxic to many bacteria, fungi, protozoa, and higher organisms. The pKa of the carboxyl group in monensin is 7.95, so at the acidic pH of the rumen, the uncharged lipophilic molecule accumulates in cell membranes of bacteria sensitive to this ionophore. Monensin forms cyclic complexes with alkali metal cations (Na ion, K ion, Rb ion) with a preference for Na ion, with six oxygen atoms serving as ligands to the cation. The ratio of Na ion / K ion
concentrations in the rumen ranges from 2 to 10. The direction of metal ion and proton movement across a cell membrane is directed by the magnitude of the existing ion concentration gradient. Monensin acts as an “antiporter” that releases a proton at the inner face of the cytoplasmic membrane as it picks up K ion
At the outer face of the cytoplasmic membrane, it releases the K ion and picks up either H ion or Na ion. The cell responds to these ion fluxes by utilizing its Na/K and H ion ATPase's to maintain ion balance and intracellular pH. Depending on the extent of exhaustion of ATP, and of the resulting membrane depolarization, the cells cease to grow and reproduce, and may die.
In the anaerobic environment of the rumen, ruminal microorganisms generate the energy and nutrients for their growth by fermenting carbohydrates (primarily cellulose) and proteins. The major resulting products, volatile fatty acids (acetic, propionic, and butyric) and microbial protein, serve as the sources of
energy and nutrients for the cow.The fatty acids pass through the rumen wall into the bloodstream. The cow
derives most of its energy from the oxidation of these compounds. Degradation of the microbial cells in the gastrointestinal tract provides amino acids. However, other bacterial fermentation end products, particularly methane and ammonia that are released to the environment, represent loss to the cow of a sizeable fraction of the potential energy and protein sources from the feed. The major end products of the fermentative metabolism of the Gram positive bacteria in the rumen are acetate, butyrate, formate, lactate, hydrogen, and ammonia. The methanogenic bacteria in the rumen are not able to use complex organic compounds. They obtain energy by utilizing formate, acetate, carbon dioxide, and hydrogen to generate methane.
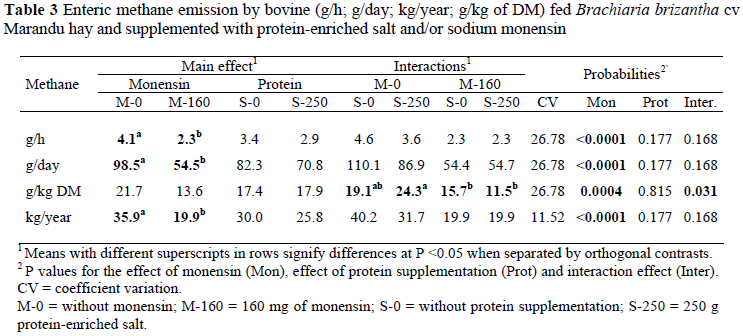
The effects of monensin on ruminal fermentation are as follows. Much less methane is produced. The ratio of propionate to acetate is higher. Less ammonia is produced, and the amount of protein N available to the cow is greater. How does monensin modulate the fermentative metabolism in the rumen?
The recommended daily dosage of monensin is 350 mg, the mass of the
monensin–Na+ The recommended daily dosage of monensin is 350 mg, the mass of the monensin–Na+ complex is 693 , and the rumen volume of cattle is approximately 70 L. Thus, the initial ruminal concentration of unbound monensin–Na+ is 7µM. At such a low concentration, monensin–Na+
rapidly partitions into the membranes of the most sensitive bacteria.
However, studies with radio labeled monensin show that binding also takes place to feed particles,
protozoa, and ionophore-resistant bacteria. The potential binding sites are
far from saturated at this monensin concentration. Gram-positive ruminal bacteria are more sensitive to monensin than are Gram-negative ones. In general, bacteria with outer membranes and/or associated extra cellular polysaccharide are more resistant, presumably because of the hindrance of access of monensin to the cytoplasmic membrane. Under these conditions, monensin does not inhibit methanogenic bacteria but does inhibit the Gram-positive H2-producing bacteria that supply the methanogens with H2 and that also produce acetate, butyrate, and formate. The result is a decrease in methane production. The fermentative pathways of ruminal Gram-negative bacteria lead to propionate and succinate. These organisms are not inhibited by monensin. The overall result is an increase in organisms are not inhibited by monensin. The overall result is an increase in the propionate-to-acetate ratio, in essence an increase in the energy source
for the cow. The ruminal obligate amino acid–fermenting bacteria are monensin sensitive. The inhibition of
these bacteria produces the large observed decrease in ammonia production. The consequence is that more protein N is available to the cow.
In summary, monensin modulates ruminal fermentative metabolism by
selective inhibition of the metabolic activities of particular groups of bacteria. However, studies with
radio labeled monensin show that binding also takes place to feed particles,
protozoa, and ionophore-resistant bacteria. The potential binding sites are
far from saturated at this monensin concentration. Gram-positive ruminal bacteria are more sensitive to monensin than are Gram-negative ones.
In general, bacteria with outer membranes and/or associated extra cellular polysaccharide are more resistant, presumably because of the hindrance of access of monensin to the cytoplasmic membrane.
Under these conditions, monensin does not inhibit methanogenic bacteria but does inhibit the Gram-positive H2-producing bacteria that supply the methanogens with H2 and that also produce acetate, butyrate, and formate. The result is a decrease in methane production. The fermentative pathways of ruminal Gram-negative bacteria lead to propionate and succinate . These organisms are not inhibited by monensin. The overall result is an increase in the propionate-to-acetate ratio, in essence an increase in the energy source
for the cow. The ruminal obligate amino acid–fermenting bacteria are monensin sensitive. The inhibition of these bacteria produces the large observed decrease in ammonia production. The consequence is that more protein N is available to the cow.
In summary, monensin modulates ruminal fermentative metabolism by selective inhibition of the metabolic activities of particular groups of bacteria.
Cited By Kamal Singh Khadka/ Shailendra Parajuli
Msc Microbiology, TU.
Assistant Professor In Pokhara University, Pokhara Bigyan Thata Prabidhi Campus, PNC, LA, NA.
Pokhara, Nepal.
Some Suggested References:
www.biolegend.com/monensin-solution-1000x-1500.html
en.wikipedia.org/wiki/Monensin
www.journalofanimalscience.org/content/43/3/670.full.pdf
www.fda.gov/AnimalVeterinary/Products/.../ucm129991.htm
www.elanco.us/products-services/beef/rumensin-p.aspx
www.ncbi.nlm.nih.gov › Journal List › Can Vet J › v.46(10); Oct 2005
www.ncbi.nlm.nih.gov/pubmed/2160275
www.ncbi.nlm.nih.gov/pubmed/6378867
aac.asm.org/content/55/2/745
journalofanimalscience.org/content/58/6/1518.full.pdf+html
.
.
MONENSIN
Monensin is the most widely used compound fed to cattle to increase feed
efficiency. In feedlot cattle, a dosage of 350 mg/day led to an improvement
in feed efficiency of approximately 6%. In grazing cattle, the average daily
gain increased by 15%. Monensin produces these outcomes by changing the
makeup of the bacterial population in the rumen, thereby influencing the
balance of the end products of ruminal fermentation metabolism.
Monensin is produced by the bacterium Streptomyces cinnamonensis. It is a member of a large and important class of polyketides, the poly ether ionophores. The compound is toxic to many bacteria, fungi, protozoa, and higher organisms. The pKa of the carboxyl group in monensin is 7.95, so at the acidic pH of the rumen, the uncharged lipophilic molecule accumulates in cell membranes of bacteria sensitive to this ionophore. Monensin forms cyclic complexes with alkali metal cations (Na ion, K ion, Rb ion) with a preference for Na ion, with six oxygen atoms serving as ligands to the cation. The ratio of Na ion / K ion
concentrations in the rumen ranges from 2 to 10. The direction of metal ion and proton movement across a cell membrane is directed by the magnitude of the existing ion concentration gradient. Monensin acts as an “antiporter” that releases a proton at the inner face of the cytoplasmic membrane as it picks up K ion
At the outer face of the cytoplasmic membrane, it releases the K ion and picks up either H ion or Na ion. The cell responds to these ion fluxes by utilizing its Na/K and H ion ATPase's to maintain ion balance and intracellular pH. Depending on the extent of exhaustion of ATP, and of the resulting membrane depolarization, the cells cease to grow and reproduce, and may die.
In the anaerobic environment of the rumen, ruminal microorganisms generate the energy and nutrients for their growth by fermenting carbohydrates (primarily cellulose) and proteins. The major resulting products, volatile fatty acids (acetic, propionic, and butyric) and microbial protein, serve as the sources of
energy and nutrients for the cow.The fatty acids pass through the rumen wall into the bloodstream. The cow
derives most of its energy from the oxidation of these compounds. Degradation of the microbial cells in the gastrointestinal tract provides amino acids. However, other bacterial fermentation end products, particularly methane and ammonia that are released to the environment, represent loss to the cow of a sizeable fraction of the potential energy and protein sources from the feed. The major end products of the fermentative metabolism of the Gram positive bacteria in the rumen are acetate, butyrate, formate, lactate, hydrogen, and ammonia. The methanogenic bacteria in the rumen are not able to use complex organic compounds. They obtain energy by utilizing formate, acetate, carbon dioxide, and hydrogen to generate methane.
The effects of monensin on ruminal fermentation are as follows. Much less methane is produced. The ratio of propionate to acetate is higher. Less ammonia is produced, and the amount of protein N available to the cow is greater. How does monensin modulate the fermentative metabolism in the rumen?
The recommended daily dosage of monensin is 350 mg, the mass of the
monensin–Na+ The recommended daily dosage of monensin is 350 mg, the mass of the monensin–Na+ complex is 693 , and the rumen volume of cattle is approximately 70 L. Thus, the initial ruminal concentration of unbound monensin–Na+ is 7µM. At such a low concentration, monensin–Na+
rapidly partitions into the membranes of the most sensitive bacteria.
However, studies with radio labeled monensin show that binding also takes place to feed particles,
protozoa, and ionophore-resistant bacteria. The potential binding sites are
far from saturated at this monensin concentration. Gram-positive ruminal bacteria are more sensitive to monensin than are Gram-negative ones. In general, bacteria with outer membranes and/or associated extra cellular polysaccharide are more resistant, presumably because of the hindrance of access of monensin to the cytoplasmic membrane. Under these conditions, monensin does not inhibit methanogenic bacteria but does inhibit the Gram-positive H2-producing bacteria that supply the methanogens with H2 and that also produce acetate, butyrate, and formate. The result is a decrease in methane production. The fermentative pathways of ruminal Gram-negative bacteria lead to propionate and succinate. These organisms are not inhibited by monensin. The overall result is an increase in organisms are not inhibited by monensin. The overall result is an increase in the propionate-to-acetate ratio, in essence an increase in the energy source
for the cow. The ruminal obligate amino acid–fermenting bacteria are monensin sensitive. The inhibition of
these bacteria produces the large observed decrease in ammonia production. The consequence is that more protein N is available to the cow.
In summary, monensin modulates ruminal fermentative metabolism by
selective inhibition of the metabolic activities of particular groups of bacteria. However, studies with
radio labeled monensin show that binding also takes place to feed particles,
protozoa, and ionophore-resistant bacteria. The potential binding sites are
far from saturated at this monensin concentration. Gram-positive ruminal bacteria are more sensitive to monensin than are Gram-negative ones.
In general, bacteria with outer membranes and/or associated extra cellular polysaccharide are more resistant, presumably because of the hindrance of access of monensin to the cytoplasmic membrane.
Under these conditions, monensin does not inhibit methanogenic bacteria but does inhibit the Gram-positive H2-producing bacteria that supply the methanogens with H2 and that also produce acetate, butyrate, and formate. The result is a decrease in methane production. The fermentative pathways of ruminal Gram-negative bacteria lead to propionate and succinate . These organisms are not inhibited by monensin. The overall result is an increase in the propionate-to-acetate ratio, in essence an increase in the energy source
for the cow. The ruminal obligate amino acid–fermenting bacteria are monensin sensitive. The inhibition of these bacteria produces the large observed decrease in ammonia production. The consequence is that more protein N is available to the cow.
In summary, monensin modulates ruminal fermentative metabolism by selective inhibition of the metabolic activities of particular groups of bacteria.
Cited By Kamal Singh Khadka/ Shailendra Parajuli
Msc Microbiology, TU.
Assistant Professor In Pokhara University, Pokhara Bigyan Thata Prabidhi Campus, PNC, LA, NA.
Pokhara, Nepal.
Some Suggested References:
www.biolegend.com/monensin-solution-1000x-1500.html
en.wikipedia.org/wiki/Monensin
www.journalofanimalscience.org/content/43/3/670.full.pdf
www.fda.gov/AnimalVeterinary/Products/.../ucm129991.htm
www.elanco.us/products-services/beef/rumensin-p.aspx
www.ncbi.nlm.nih.gov › Journal List › Can Vet J › v.46(10); Oct 2005
www.ncbi.nlm.nih.gov/pubmed/2160275
www.ncbi.nlm.nih.gov/pubmed/6378867
aac.asm.org/content/55/2/745
journalofanimalscience.org/content/58/6/1518.full.pdf+html
.
.






Comments